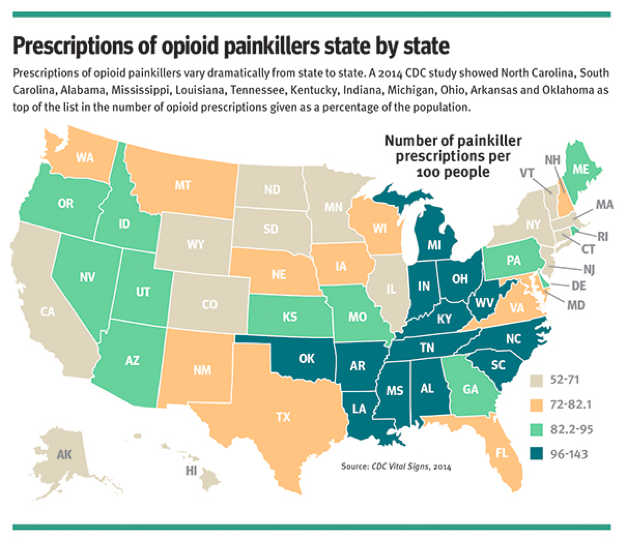
Webinar Q and A: Opioid Prescription Drug Abuse
First Healthcare Compliance hosted an educational webinar, “Risk Management Considerations with Prescription Drug Abuse” with Mike Midgley RN, JD, MPH, CPHRM, DFASHRM. Click here to view the webinar.
For prescribers, dispensers and their employees, Mike provides answers to some commonly asked questions regarding risks related to prescription drugs.
Q: What is the best way to assess liability issues with a pain clinic?
A: I recommend using an enterprise risk management (ERM) approach to assess risks in eight (8) areas: clinical/patient safety, legal/regulatory, human capital, technology, operations, hazard, strategic and financial. Risks are ranked according to their severity, likelihood and velocity to come up with a ranked risk inventory. Priority should be given to addressing the most significant risks.
Q: What is the most critical strategy for reducing prescription opioid abuse?
A: The prescription drug monitoring programs (PDMPs) in each state need to be mandatory for each prescriber and dispenser to enter prescription information for every patient prescribed opioids to include every time an opioid is prescribed and renewed. Prescribers and dispensers need to be required to query the database each time a patient is prescribed or given a renewal for opioids. This will eliminate patients being prescribed multiple opioids from various prescribers. 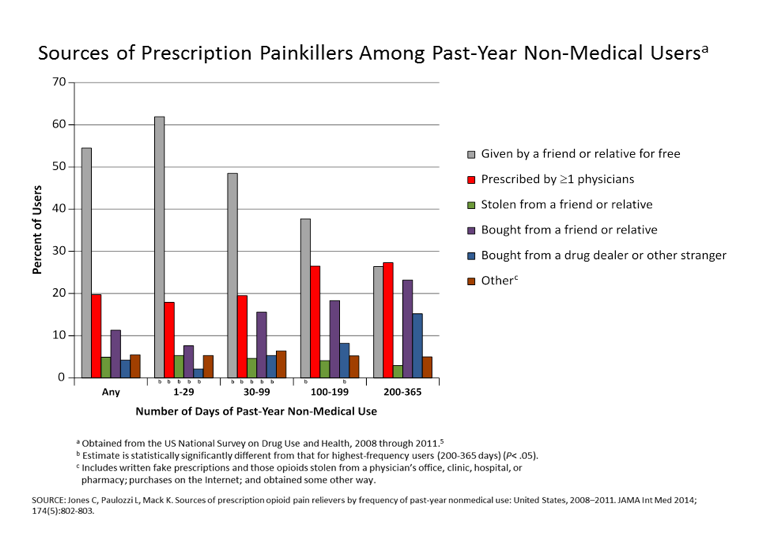
Q: Should all patients on chronic opioids be required to submit to a urine drug test on a routine basis?
A: After completing an abuse risk assessment of the patient and assigning the patient into an abuse risk class (low, medium or high) the prescriber should determine if and when a urine drug screen is necessary. Look to the evidence based medicine clinical guidelines for specific recommendations on the stratification of risks for patients maintained on chronic opioid therapy. Generally, your high risk patients need more monitoring and repeat assessments.